Round 2 Stem is learned about the periodic table and chemical bonding. Let me tell you about the periodic table, the periodic table was created by Dmitri Mendeleev in 1869 in Russia. His periodic table is arranged by atomic mass (because that time there are know the atomic number) this is how the old periodic table look.
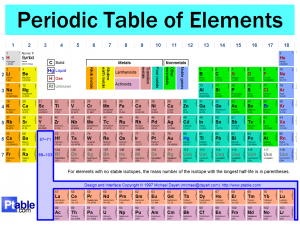
New periodic table the is arranged by atomic number. The new table looks fancier than the old one it has color and it easy. Here is the new one.
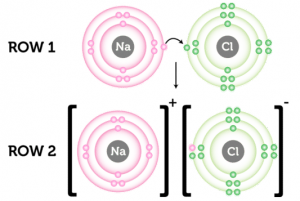
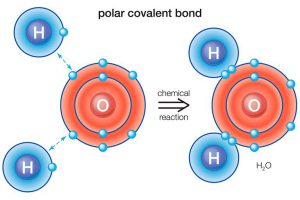
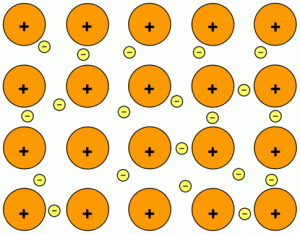
Chemical bonding is the force of attraction between atoms or ions that occurs when atoms share or transfer valence electrons. There is two type of chemical bonding, Ionic bonding, Covenlen bonding and, Metallic bonding.
Ionic bonding: Ionic bonding is transformed electron from one element to another element to make it stable.
Covenlen bonding: Covenlen bonding is to share the electron from one element to another element to make it stable.
Metallic Bonding: A metallic bond is the force of attraction between a positive metal ion and the valence electrons it shares with other ions of the metal.